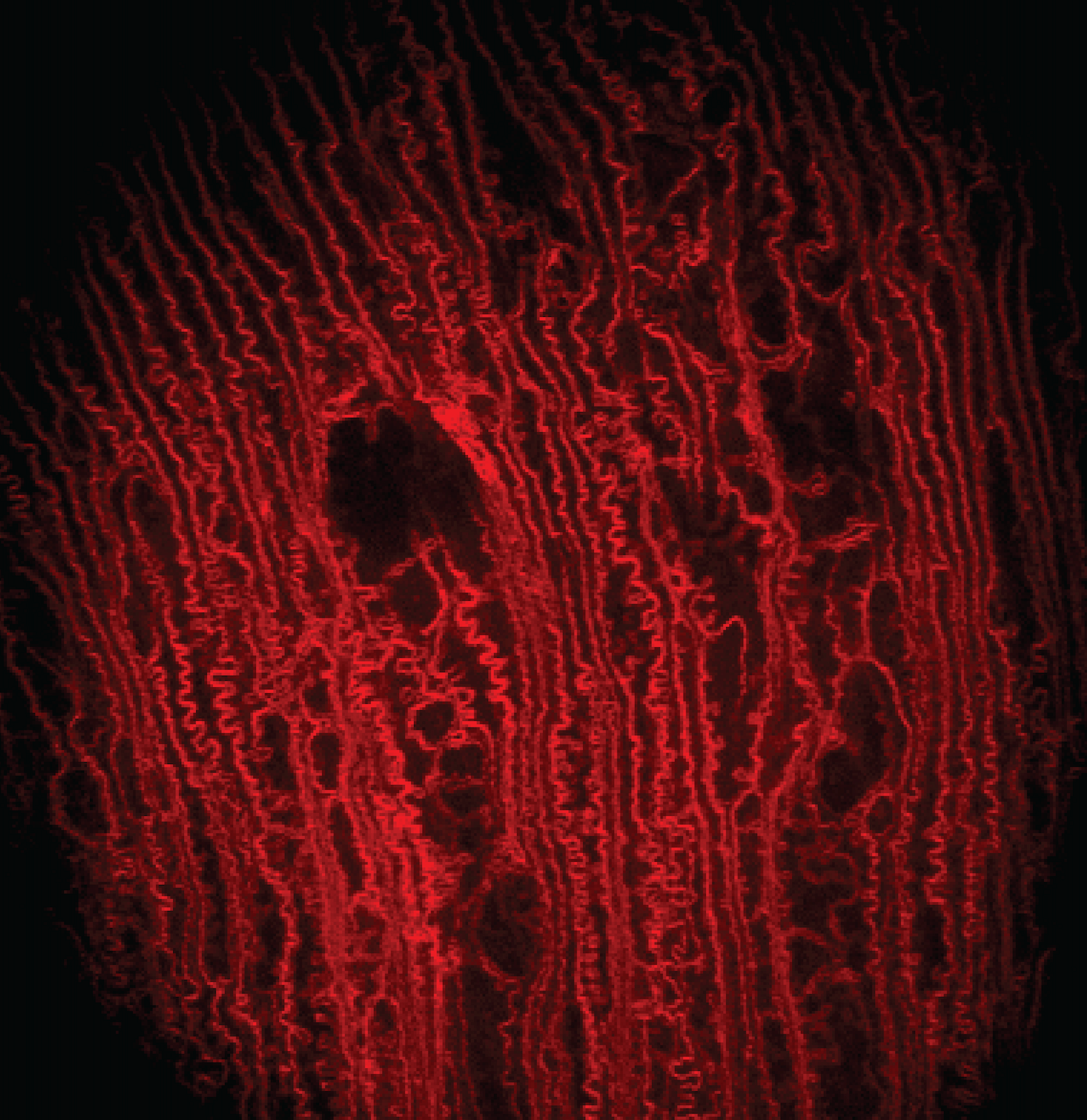
In vivo imaging of ocular lenses with adaptive optical two photon fluorescence microscopy
My postdoctoral training at the University of California, Berkeley was focused on the development of two photon fluorescence microscopy (TPFM) and adaptive optics for in vivo imaging of ocular lenses in genetic mouse models. Traditionally, microscopic studies of the cellular organization of mammalian lenses have been carried out ex vivo on dissected lenses, which eliminates both in vivo environment for supporting lens internal circulation and attached zonules for regulating lens accommodation. Our work has demonstrated that two photon fluorescence microscopy and adaptive optics can enable in vivo visualization of lens cells in transgenic mice that express fluorescent proteins in lens cell membranes. We employed direct wavefront sensing to measure and correct aberrations to image cellular organization deep inside the anterior part of the lens and discovered novel features in different regions up to near the lens core that have not been observed in prior ex vivo studies. These studies demonstrated the importance of in vivo imaging in preserving the native biological context in live animals and opened a new route for observing changes in lens cell morphology during normal development and pathological processes.
Paidi SK et al., Investigative Ophthalmology and Visual Science (iOVS), 2023